

Lead, for example, has such a low melting point that it's easily liquefied by a flame. Melting and boiling points in the carbon family tend to decrease moving down the group, mainly because atomic forces within the larger molecules are not as strong. Group 14 (carbon family) elements have much higher melting points and boiling points than the group 13 elements.Lead is only found as a dense blue-gray metal. Tin occurs as white tin, gray tin, and rhombic tin. Carbon, for example, occurs in diamond, graphite, fullerene, and amorphous carbon allotropes. Except for lead, all of the carbon family elements exist as different forms or allotropes.The elements tend to form covalent compounds, though tin and lead also form ionic compounds.Overall, the carbon family elements are stable and tend to be fairly unreactive.The carbon family elements have widely variable physical and chemical properties.Bonding configurations are readily predicted by valence-shell electron-pair. Carbon is the only element in the group that can be found pure in nature. Carbon has 4 valence electrons and oxygen has 6, for a total of 10 electrons. there is one valence electron for the seventh column there are seven. These elements are found in a wide variety of compounds. The assignment of electrons, at least for the first 20 elements with atomic.In other words, the elements gain metallicity moving down the group. The carbon family consists of one nonmetal (carbon), two metalloids (silicon and germanium), and two metals (tin and lead).Element density increases moving down the group.

CARBON VALENCE ELECTRONS PLUS
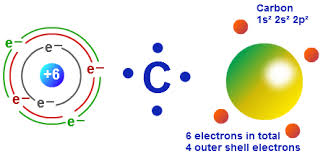
The second electron shell, 2n, contains another spherical s s s s orbital plus three dumbbell-shaped p p p p orbitals, each of which can hold two electrons. Home Carbon, silicon and germanium have four valence electrons each. Hydrogen and helium are the only two elements that have electrons exclusively in the 1 s 1s 1 s 1, s orbital in their neutral, non-charged, state. On the periodic table, hydrogen and helium are the only two elements in the first row, or period, which reflects that they only have electrons in their first shell. This is written out as 1 s 2 1s^ 2 1 s 2 1, s, squared, referring to the two electrons of helium in the 1 s 1s 1 s 1, s orbital. Helium has two electrons, so it can completely fill the 1 s 1s 1 s 1, s orbital with its two electrons. This can be written out in a shorthand form called an electron configuration as 1 s 1 1s^ 1 1 s 1 1, s, start superscript, 1, end superscript, where the superscripted 1 refers to the one electron in the 1 s 1s 1 s 1, s orbital. Hydrogen has just one electron, so it has a single spot in the 1 s 1s 1 s 1, s orbital occupied.

The 1 s 1s 1 s 1, s orbital is the closest orbital to the nucleus, and it fills with electrons first, before any other orbital. The first electron shell, 1n, corresponds to a single 1 s 1s 1 s 1, s orbital.


 0 kommentar(er)
0 kommentar(er)
